LAB HOURS: MON - THU and SAT : 9:00 AM to 9:00 PM
FRI and SUN : 9:00 AM to 6:00 PM

Article - Subfertility Assessment - 01/2016
FML at EOFF 2016 in Dubai, 01/2016

Subfertility/Infertility Assessment in the Medical Laboratory
PD Dr. med. habil. Michaela Jaksch, Senior Consultant Laboratory, Medicine Medical Director
Freiburg Medical Laboratory ME LLC, Dubai, UAE
Abstract
Literature concerning a reduced reproductive health show a confusing high number of correlation with many factors such as epigenetics, vitamin deficiencies and malnutrition, obesity and lifestyle in general, monogenic disorders, immunologic disorders such as autoimmune disorders, advanced age and many more.
Around 15% of couples experience 'subfertility' during the first year of intercourse. In addition, reproductive failures such as implantation failures and repeated miscarriages, but also malformation and fetal growth restriction must be considered. Evidence based laboratory diagnostics are needed to support successful outcomes in these couples.
This article focuses on several algorithms which can be used to further assess subfertility/infertility. As an example, malnutrition is prevalent in developing but also in developed countries. Metabolic pathways and cellular physiological functions rely on healthy nutrition. A prominent pathway is the one-carbon methylation cycle requiring folate, vitamin B12, vitamin B6 and an intact methylenetetrahydrofolate reductase (MTHFR) enzyme. Deficiencies or imbalances might result in hyperhomocysteinemia, which has been associated with reproductive failures, miscarriages, congenital malformation and fetal growth restriction.
Further examples will focus on autoimmune disorders and on recent genetic approaches.
Introduction
Pubmed (www.ncbi.nlm.nih.gov/pubmed/) shows 39000 articles when searching for the causes of subfertility or infertility. The probably most accurate definition of infertility and subfertility is provided by Gnoth et al. (2005) as well as by Habbema et al. (2004).
Definition and Prevalence of Subfertility and Infertility
Table 1 (according to Gnoth et al., 2005)
|
Time |
Prevalence/Grading |
Chances to Conceive Spontaneously in the Future |
|
After 6 unsuccessful cycles |
About 20% at least slightly subfertile couples |
50% of these couples will conceive spontaneously in the next six cycles, the remaining are moderately subfertile [Equivalent to slightly reduced fertility (Habbema et al., 2004)] |
|
After 12 unsuccessful cycles (WHO clinical definition of in-fertility) |
About 10% at least moderately or seriously subfertile couples |
50% of these couples will conceive spontaneously in the next 36 months, the remaining are nearly complete infertile [Equivalent to moderately/seriously reduced fertility, (Habbema et al., 2004)] |
|
After 48 months |
About 5% nearly complete infertile couples |
Couples with only sporadic spontaneous conceptions [Equivalent to sterile couple (Habbema et al., 2004)] |
What are the main causes of subfertility and infertility?
Male and female factors are most probably equally distributed.
According to the definition outlined above, subfertility is a less severe reproductive health problem, than infertility. Sperm DNA fragmentation, autoimmune disorders (for review 'autoimmune disorders': see Jaksch M., EOFF Journal 2014 and for review 'sperm fragmentation' EOFF Journal, this issue) , metabolic syndrome and increased BMI (Chavarro et al., 2010), environmental exposures to toxins (Meeker et al., 2010), infections (Lal et al., 2013), suspected implantation failure (Kwak-Kim et al., 2014) and polycystic ovary syndrome (PCOS; Conway et al., 2014) are factors associated with subfertility.
Monogenic factors are more likely to be involved in infertility, however genetic factors contribute to unclear subfertility as well.
Although male factors account for approximately 50% of all infertility, the mechanisms underlying their origin are unknown. Currently, clinicians rely primarily on semen analyses to predict male reproductive potential and chart treatment success. Even when invasive procedures are performed, the causes of male factor infertility frequently remain elusive (Kovac et al., 2013). Recent estimates indicate that genetic abnormalities cause 15-30% of male factor infertility (Ferlin et al., 2007). For example, spermatogenesis involves the aggregated action of up to 2300 genes, any of which, could, potentially, provide targets for diagnostic tests of male factor infertility (Hotaling and Carrell, 2014). The same holds true for the unclear female factors. Genes involved in ovarian and endometrial reproductive functional interaction are expected > 5000.
What are the Best Practical Approaches for the Clinician in order to Assess Subfertility/Infertility?
There are many causes in both, female and male which can be diagnosed and treated without a major involvement of the laboratory (undescended testicles, diabetes, varicoceles, lifestyle, uterine or cervical abnormalities, tumors, tube damage or blockage, endometriosis etc.).
Some disorders need a basic laboratory assessment (autoimmune disorders, thyroid disorders, coagulation disorders, vitamin deficiencies etc.) The challenge however are always the unclear cases.
Patient's history, family history, clinical examination of the couple, basic blood tests and hormone profiles are usually performed for the first assessment.
The basic blood screen with full blood count and differential will allow to pre-assess possible hemoglobinopathies, iron or vitamin B12 and/or folate deficiencies. However, this might be difficult in carriers of hemoglobinopathies, as indices could be masked. We recommend to test for Ferritin and Vitamin B12 in every woman/man with possible subfertility/infertility (Forges et al., 2007). In this context it is of interest that a positive correlation of seminal methylcobalamin (Vitamin B12) with sperm concentration has been described (Boxmeer et al., 2007).
In addition the blood differential allows a pre-assessment of a possible infectious disorder. CRP, high vaginal and urethral swabs for culture and for STDs are mandatory tests in order to detect possible infections.
In all women with subfertility/infertility, Anti-Muellerian Hormone (AMH) testing is of central importance. This marker has a high potential in assessing ovarian response, menopause prediction and PCOS. It is lowered in premature ovarian insufficiency or failure (POF/POI), in autoimmune disorders and in contraceptive supplementation (for review see Leader and Baker, 2014). As with the AMH in women, semen analysis in men (according to WHO 5th edition) is considered the next step. Furthermore, Inhibin B levels are higher in men with apparently normal fertility than in those with sub/ infertility and abnormal spermatogenesis. Serum Inhibin B should be used in combination with FSH, as a more sensitive marker of spermatogenesis than FSH alone (Grunewald et al., 2013). However, the optimal level of inhibin B to assess male infertility has not been established.
Karyotyping in both, husband and wife in order to exclude balanced translocations and other abnormalities should be done next. In addition, screening of Y-deletions and CFTR mutations is recommended in cases of oligospermia/azoospermia (Stahl and Schlegel, 2012). Sperm aneuploidy testing using FISH should be considered as well, especially if the sperm show abnormal morphology, such as round-headed sperm syndrome or micro- and macrocephaly (Hotaling and Carrell, 2014). In order to exclude autoimmune processes and coagulation disorders, I recommend to perform ANA screening, TPO and TG antibody testing as well as Factor II and V mutation screening, PT/INR and aPTT, Protein C, S and Antithrombin testing.
Sperm DNA fragmenation testing should be considered next if all previous tests and examination were normal. In case of suspected repeated implantation failures cellular and humoral tests can be performed.
If the cause is still not established, genetic panel testing (>100 genes) or even the whole exome sequencing can be recommended. Due to newer technologies (high throughput DNA sequencing, next generation sequencing) allowing fast sequencing of thousands of genes (the human exome consists out of 30.000.000 basepairs and around 180.000 exons) the cost is not the limiting factor anymore (see figures 1-3).
Lifestyle and Nutrition
As outlined in the abstract, lifestyle and nutrition as major factors for obesity, diabetes, malnutrition despite overweight, might play an underestimated role in subfertility. There is known relationship between body weight and reproductive hormones. For example, obesity is associated with increased sperm DNA damage (Chavarro et al., 2010).
The methylation cycle (requiring Folate, Vitamin B6, B12, MTHFR) is of central importance for many physiological processes. There is a growing body of evidence demonstrating a relationship between Folate and other B Vitamin deficiencies, hyperhomocysteinemia and gonadal abnormalities, such as altered spermatogenesis and impaired ovarian reserve, as well as male and female infertility. However, the exact mechanisms underlying these phenomena still need to be further investigated (Forges et al., 2007).
In the UAE, we experience a very high number of Vitamin D deficient patients. Repeated correlation - however conflicting - of Vitamin D deficiency and reduced pregnancy outcomes have been described (Polyzos et al., 2014). Vitamin B12 deficiency, often combined with iron deficiency is found frequently in all UAE ethnicities as well. To which extent malnutrition and Vitamin deficiencies play an important role in reproductive health is controversially discussed in the literature, however diseases, environmental factors and nutrition have an important impact on epigenetics. Epigenetic modifications including DNA methylation, histone acetylation and RNA interference are involved in functional changes in pregnancy (Altmaee et al., 2014).
Summary and Perspectives
The Medical Laboratory can support the Clinicians in unclear cases of subfertility/infertility. Newer developments in Genetics allow a broader and more detailed assessment of unresolved cases.
Figure 1: Steps in NGS Technology
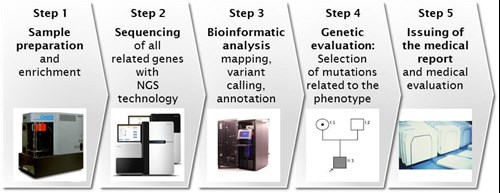
Figure 2: From the Nucleus to the Genes and Proteins
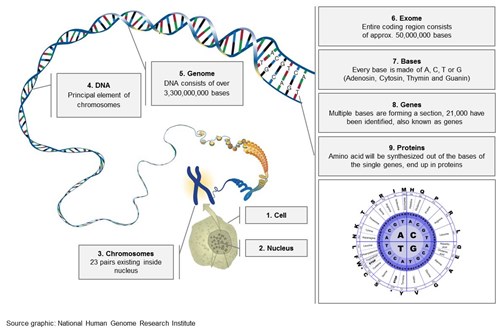
Figure 3: Next Generation Sequencing Equipment

Literature
Altmaee et al. (2014) Guidelines for the design, analysis and interpretation of 'omics' data: focus on human endometrium. Hum Reprod Update; 20(1):12-28
Boxmeer et al. (2007) Seminal plasma cobalamin significantly correlates with sperm concentration in men undergoing IVF or ICSI procedures. J Androl; 28(4):521-527
Broer et al. (2014) Anti-Muellerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update; 20(5):688-701
Chavarro et al. (2010) Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril; 93:7:2222-2231
Conway et al. (2014) The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol; 171(4):P1-29
Ferlin et al. (2007) Male infertility: role of genetic background. Reprod Biomed Online; 14(6):734-745
Forges et al. (2007) Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update; 13(3):225-238
Gnoth et al. (2005) Definition and prevalence of subfertility and infertility. Hum Reprod; 20(5):1144-1147
Grunewald et al. (2013) Age-dependent inhibin B concentration in relation to FSH and semen sample qualities: a study in 2448 men. Reproduction; 145(3): 237-244
Habbema et al. (2004) Towards less confusing terminology in reproductive medicine: a proposal. Hum Reprod; 19(7):1497-1501
Hotaling and Carrell (2014) Clinical genetic testing for male factor infertility: current applications and future directions. Andrology; 2(3):339-350
Kovac et al. (2013) The use of genomics, proteomics, and metabolomics in identifying biomarkers of male infertility. Fertil Steril; 99(4):998-1007
Kwak-Kim et al. (2014) Immunological modes of pregnancy loss: inflammation, immune effectors, and stress. Am J Reprod Immunol; 72(2):129-140
Lal et al. (2013) Chlamydia trachomatis infections and subfertility: opportunities to translate host pathogen genomic data into public health. Public Health Genomics; 16(1-2):50-61
Leader and Baker (2014) Maximizing the clinical utility of antimuellerian hoemone testing in women's health. Curr Opin Obstet Gynecol; 26(4):226-236
Meeker et al. (2010) Environmental exposure to metals and male reproductive hormones: circulating testosterone is inversely associated with blood molybdenum. Fertil Steril; 93:1: 130-140
Polyzos et al. (2014) Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod; 29(9):2032-2040
Stahl and Schlegel (2012) Genetic evaluation of the azoospermic or severely oligozoospermic male. Curr Opin Obstet Gynecol; 24(4):221-228

Lab hours
MON - THU and SAT 9:00 AM to 9:00 PM | FRI and SUN : 9:00 AM to 6:00 PM













